Michael Pendleton: Researchers Have Created the Lining of a Womb in a Dish
Michael Pendleton, Professor Emeritus of Law, shared a post on LinkedIn:
“Absolutely Fascinating, and a reminder from science that there’s no such thing as alternative facts.
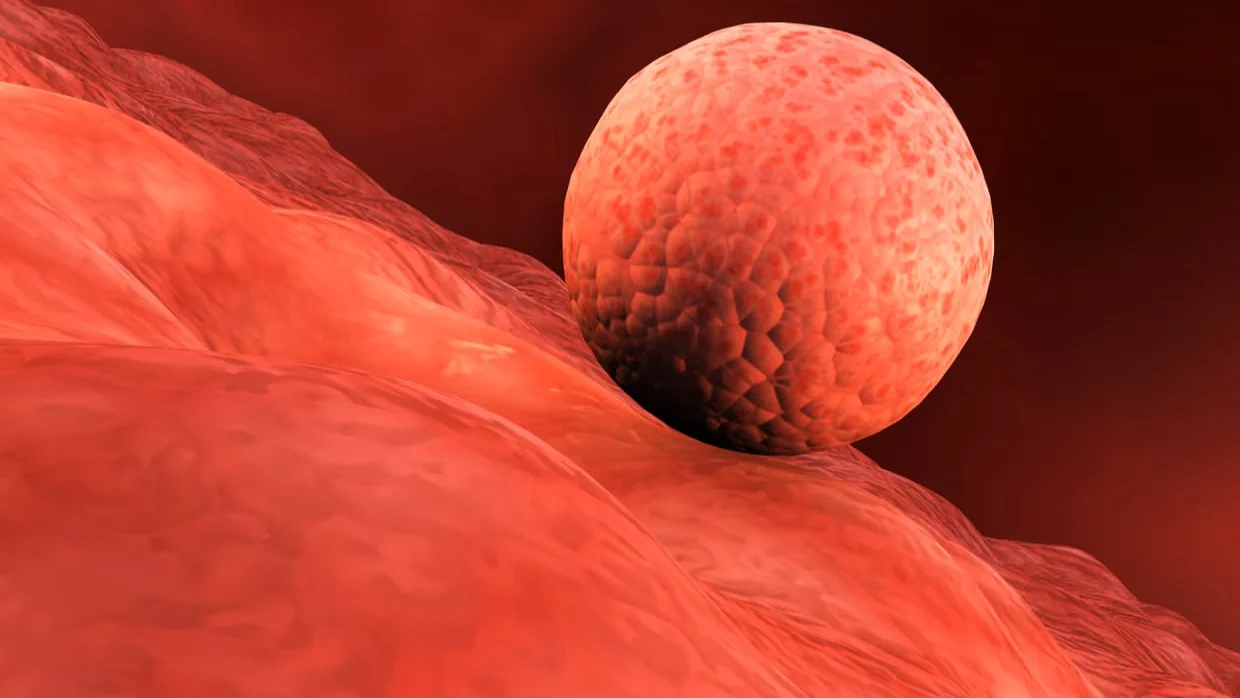
‘Researchers have created the lining of a womb in a dish, which promises to shed light on the mysterious early stages of human pregnancy and the glitches that can lead to miscarriage and medical complications.
In laboratory experiments, early-stage human embryos donated from couples after IVF treatment successfully implanted into the engineered lining and began to churn out key compounds, such as the hormone that results in a blue line on positive pregnancy tests.
The approach allowed scientists to eavesdrop on the chemical chatter that arises between the embryo and the womb lining as it embeds and begins to be nourished in the first weeks of gestation.
‘It’s incredible to see it,’ said Dr Peter Rugg-Gunn, a senior author on the study and group leader at the Babraham Institute in Cambridge. ‘Previously we’ve only had snapshots of this critical stage of pregnancy. This opens up a lot of new directions for us.’
Implantation takes place a week or so after fertilisation when the developing embryo attaches and then embeds itself into the uterus wall. It is a crucial phase in pregnancy, but the process is poorly understood because it is so hard to observe. Much of what is known comes from studies of hysterectomies that were performed in early pregnancy more than half a century ago.
To build the replica womb lining, Rugg-Gunn and his colleagues obtained uterine tissue from healthy women who donated biopsy samples. From this, the scientists isolated two different types of cells: stromal cells that give structural support to the womb lining, and epithelial cells, which form the lining’s surface. They encapsulated the stromal cells in a biodegradable material called a hydrogel and put the epithelial cells on top.
The researchers tested the engineered womb lining with donated early-stage IVF embryos. Writing in the journal Cell, they describe how the microscopic balls of cells attached and implanted as hoped. They then ramped up secretion of a hormone called human chorionic gonadotropin (hCG), the biochemical marker detected by pregnancy tests, along with other pregnancy-related compounds.
The technique enabled scientists to watch embryos grow for up to 14 days post-fertilisation, the legal limit for research. During the process, the embryos formed specialised cells and other cells involved in the growth of the placenta.’ ”
Stay updated on all scientific advances in the field of fertility with Fertility News.
-
Oct 11, 2025, 06:44The Global IVF Market Is Set to Reach $65B by 2032 – Meddilink
-
Mar 3, 2026, 00:23Final Call for Review of Updated ESHRE IVF Laboratory Recommendations – ESHRE
-
Mar 3, 2026, 00:15Abstract Submissions Are Now Open for ASM 2026! – BSGI
-
Mar 2, 2026, 14:06Ever Wondered How Ovulation Works? Let’s Break It Down! – Fertility Plus
-
Mar 2, 2026, 13:48Join RBMO Webinar on Global Declining Fertility Rates – RBMO
-
Mar 2, 2026, 13:39Governance of Polygenic Embryo Screening – Fertility and Sterility
-
Mar 2, 2026, 13:26AJOG MFM February 2026 Podcast Episode Is Out!
-
Mar 2, 2026, 13:21Yousef Zakharia Leads Clinical Research Aimed at Genitourinary Cancers – Mayo Clinic
-
Mar 1, 2026, 16:41Exploring Frozen and Fresh Embryo Transfer Risks – Fertility and Sterility
-
Mar 1, 2026, 16:40Foraviset Welcomes Prof. Tasuku Harada to Advisory Board – Foraviset
